First of all, I should mention that I am not a radiation expert, and have gathered this information via the internet. This in an attempt to understand the medical treatment with RAI that I have undergone in a logical context. I also wanted to present this topic to other laymen in easy to understand language. The content of this article may however be corrected, or new information can be added in the future.
Radium
As you may have gathered from -RAI 1 My Story-, I am not a fan of medical treatment with radioactive radiation.
But there is another type of radium radiation that I absolutely adore, and that is the sun!
I LLLLOOOOOOVVVVVVEEEEEEE IT!
Okay and now a bit technical…
Radio Active Iodine
This article is about the isotope Radioactive Iodine. And for those who don’t know yet, isotopes are the names of chemical elements from the periodic table.
This is also reflected in the meaning of the word. ‘Isotope’ comes from the Greek: ‘isos’ = ‘same’ and ‘topos’ = ‘place’. This indicates that different isotopes of one and the same element, in this case the substance iodine, occupy the same place in the periodic table.
Iodine Isotopes
There is only one isotope of the substance iodine that is present in a natural composition on Earth. This is described as ‘I-127’. That stands for Iodine-127. (Iodine is the English word for Iodine). In addition, 26 radioactive isotopes of iodine have been produced by humans. These are called ‘radio-isotopes’, which include I-123 and I-131. Those are two common forms of radioactive iodine that are used for medical purposes, and are therefore also described as ‘medical isotopes’.
Perhaps also interesting to mention is that the longest living man-made isotope of the element iodine is I-129, according to Wikipedia with a half-life of 15.7 million years. However can we be sure of that for such a long time to know? After which they claim it’ll decay by β decay to the stable isotope xenon-129.
Iodine
As previously written in part 1 of this article series, the ‘I’ of the abbreviation for the iodine isotopes stands for ‘Iodine’, the English word for Iodine. The English ‘I’ is usually also used as an international abbreviation in science to describe the element Radio Active Iodine, i.e. RAI, and we also use it for this article. In Dutch, Iodine is ‘Jodium’ and thus we describe RAI as RAJ.
Iodine and the Thyroid
The English word ‘Iodine’ comes from the Greek ‘iodius’ which means ‘violet’ and refers to the color of iodine when it evaporates. Iodine is a naturally occurring substance and essential for metabolism. Iodine concentrates in the thyroid gland as this organ as a kind of mini factory produces thyroid hormone using iodine. The thyroid gland does this by linking iodine and proteins from our digested food, which is absorbed into the blood, and flows through the thyroid gland.
T3 and T4
This process produces different molecular compositions of thyroid hormone, all of which have a specific effect in the body and cell metabolism. The two main ones are described as ‘T3’ and ‘T4’. However, there are more variants (T1, T2, etc.), and also in free form, for example ‘Free T3’ of ‘FT3’.
The T refers to the amino acid ‘Tyrosine’, which as a protein component forms the building block for the two types of thyroid hormone ‘Thyroxine’ (T4) and ‘Tri-iodo-thyronine’ (T3). So you have to think that T stands for the protein part of the thyroid hormone. The word ‘Thyreoiedes’ also starts with a T, it comes from Greek and means shield, based on the shape of the thyroid gland.
The number 3 or 4 after the T refers to the number of iodine atoms linked to the protein amino acid Tyrosine. The different number combinations, so to speak, also have different effects, all of which are necessary for a good metabolism. But that is another book in itself, or rather, a huge number of books have been written about the functioning of the thyroid gland and the diverse hormones it produces. Outstanding books in the field of T3 are those by Paul Robinson, such as the first book he wrote: ‘Recovering with T3’.
Calcium Metabolism
The thyroid gland also produces another substance, namely Calcitonin (helps absorption of calcium into the cells and inhibits the breakdown of bone tissue). There are also tiny parathyroid glands around the thyroid gland. These are as small as a grain of rice, but produce the important Parathormone. This hormone helps with the absorption of calcium into the bones. Both substances are therefore responsible for the calcium balance. Thyroid hormone is absorbed by all cells in the body and is concentrated in the stomach, glands with internal secretions, and other organs and tissues.
Stable Atom
Now let’s move on to the radioactive part. An atom is the smallest building block of matter, and therefore also of our tissues.
Normally in an atom, the Protons and Neutrons together form the positively charged (+) atomic nucleus. The negatively charged (-) electrons float around it in a stable state. When + and – are in balance, an atom is neutral. This means that the cell nucleus and the envelope keep each other in balance.
Radio-isotope
This is not the case with the radioisotopes of radioactive iodine, which do not occur naturally on Earth. These have been manufactured by humans and made unstable, so that the Protons and Neutrons in the cell nucleus and the Electrons around them in the shell of the atoms are no longer in balance with each other. This causes the isotopes to become radioactive. And they are of a completely different order.
However, radioactive substances such as Iodine I-131 and I-123 are used as so-called ‘medical isotopes’. Medical radioisotopes are also made from other substances (such as cobalt, erbium, iridium, gold, phosphorus, strontium, etc.), but these will not be discussed in this article as we focus on RAI).
Atomic-mass
What do the numbers 123 and 131 after the ‘I-‘ stand for? The numbers indicate the atomic mass of the chemical element. As previously written: each atom contains a cell nucleus containing protons and neutrons. Electrons float in a cloud around the mass of the cell nucleus. The number of protons in the cell nucleus determines the chemical properties of the atom, which is classified in atomic numbers. The chemical element iodine contains 53 protons in the cell nucleus, which is therefore also the atomic number of iodine.
However, the number of neutrons in the cell nucleus of different iodine substances can differ from each other. In addition to the 53 protons, I-131 contains 78 neutrons in the cell nucleus. The protons and neutrons added together form the atomic mass (53 + 78 = 131). So 131 is the total mass of the cell nucleus. In the case of I-123, the atomic mass is 123 (53 + 70 = 123). Are you still following? Yes, it took me a while too…
Iodine radio-isotopes
Various radioactive iodine isotopes such as Iodine I-131 and I-123 are created by nuclear fission of heavier elements in nuclear reactors, such as the fissile materials Uranium and Tellurium. With both substances this is done by weighing down the cell nuclei through neutron irradiation, causing the cells to become unbalanced which will then start to radiate.
If I understand correctly, the cell nuclei of other fission material can also be irradiated with protons (which changes the chemical composition). And in other substances, the electrons around the nucleus are removed by means of radiation, which also makes an atom unstable, both with the complete removal of the electrons or with part of them.
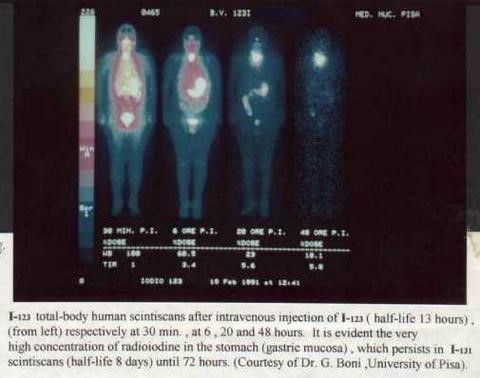
I-123
Iodine I-123 or 123I is an unstable isotope. It has a half-life of 13 hours. It is a halogen (gaseous form) and accumulates in the body in the thyroid and other iodine processing tissues. It is used for medical purposes in nuclear medicine. For example, this radioactive substance is used to detect cancer cells in the body or it is used as a low dose for a thyroid uptake scan (where the production capacity and size of the organ are measured). I-123 is administered intravenously and then traced using a special gamma camera.
Wikipedia org describes that I-123 is created from xenon-124 by irradiating it with protons. As a result, it changes into xenon-123 due to the release of a neutron and a proton, or it changes over with the release of two neutrons into caesium-123, also described as 123Cs. This caesium-123 itself also decays into xenon-123. This isotope has a half-life of 2 hours and further decays to iodine I-123.
I-131
Iodine I-131 or 131I, has a half-life of 8 days. Like sodium iodide, it is used for both diagnosis and treatment. It is an unstable radioisotope and is a waste product of nuclear fission of uranium (U-235-3%), which is produced by irradiation of the cell nuclei with neutrons. I-131 is also released during the fission of highly enriched uranium (HEU -> 20%).
According to scientists, U-235 has a half-life of 703.8 million years (but we will only find out in due course whether this time indication is really correct).The nuclide emits both beta and gamma radiation, but largely emits beta radiation for about 90%. The betas destroy, among other things, different types of (thyroid) tumor cells, and are also used for an overactive or enlarged thyroid gland.Gamma radiation is the form of radiation that makes the iodine-containing tissue visible under a gamma camera.
However, the radioactive form Iodine I-131 can, as a particle beta emitter, cause mutations in the DNA (including healthy cells), and in larger quantities can lead to death.However, according to the Laka.org foundation, the metalloid Tellurium-131 (Te-131 or 131Te) is usually used for the production of I-131, the cell nuclei which is also irradiated (or ‘bombarded’) with neutrons.
Tellurium is a brittle metallic mineral and a fairly rare element that usually occurs in nature in a bound state (such as the mineral Calaverite). However, the number of neutrons determines, among other things, the stability of the nucleus. By adding extra neutrons through radiation, the + energy increases in the cell nucleus. Instead of being neutral, it has now become an atom or molecule with a surplus of positive charge. After all, there is now more + than – energy.
Ionisation
Beta radiation is also called ionizing radiation. When an atom is out of balance, it will seek equilibrium because it can only function in neutrality. It does this by looking for extra electrons outside itself, it is, as it were, attracted to them (the opposites + and – attract each other). It wants to regain equilibrium with the increased atomic mass in the cell nucleus.
This causes it to move, but very strongly in one direction, which, simplistically explained, causes it to lose its balance. It falls over, as it were, and continues spinning at high speed in the quest for electrons. This causes friction, and therefore heat or radiation.
Now the unstable atomic nucleus has turned into what is called an ‘ion’. This is an out of balance atomic nucleus that emits pulsating electromagnetic radiation at a high frequency. This is therefore called ‘ionization’. The substance has therefore also become halogen, it is in an airy state due to the rapid movement, like a gas, because it has proportionately fewer electrons than the surplus of neutrons in the cell nucleus.
The ionization also causes friction in the surrounding atmosphere of the atom or molecule. This friction is sufficient to attract electrons from neighboring atoms through the resulting electromagnetic vacuum. If the radiation is strong enough, it will even remove the electrons from the neighboring atoms due to the strong electromagnetic attraction. This is Beta radiation, an ionizing radiation of beta particles that release electrons or positrons.
These ionizing atoms are usually very reactive and will react with any atom or molecule that happens to be nearby. With even stronger radiation, even the nucleus of neighboring cells is split. That is an ionizing radioactive chain reaction and we call it nuclear fission.
Depending on the radiation intensity and the action of ionization during decay, ionization can destroy cells (completely burn them up), damage them (cell function is reduced), but also lead to mutation (the cell is damaged but can still function, albeit in battered shape, and as such also divide or renew, but is then energetically weakened, which can cause unhealthy cell growth).
Decline
The half-life, which is the radioactive decay, depends on the strength of the radiation. The medical isotope I-131 has a half-life of 8 days. This means that after every 8 days, the radiation decreases by half. It then radiates at exactly half the radiation intensity for another 8 days, and then at a quarter of the radiation intensity, and so on, until the radiation has become stable and extinguishes. During that process, I-131 decays to another atom, the isotope xenon Xe-131, and thus becomes stable again. This is of course also a halogen, with tiny residual dust particles. I do not know how this gas behaves in the body, and whether this gas leaves the body.
I-131 and I-123 are therefore halogen ionizing substances released during nuclear fission of Uranium or Tellurium and are therefore in fact a waste product. These substances are also released during nuclear disasters (such as in 1986 near the city of Chernobyl in the Ukraine & Russia, and in 2011 in the Fukushima province of Japan). This also releases cesium Ce-134 (half-life 2 years), cesium Ce-137 (half-life 30 years) and Strontium-90 (half-life 29 years). The I-131 is also formed after the radioactive decay of tellurium-131 (Te-131 or 131Te).
C-137 en Sr-90
After the nuclear disaster in Fukushima, a high percentage of damaged thyroid glands was measured among the population. It can hinder both physical and mental growth in the physical development of children and young people. In adults, radiation causes a very wide range of associated diseases, including cancer.
Cesium-137 spreads throughout the body, but tends to accumulate in the muscles, it has an affinity with potassium. Strontium-90 mimics calcium, also competes with magnesium (they are each other’s antagonists) and accumulates more in our bones and also the spine.
Units of Measurement
There is an international system of SI units and prefixes, which has been given official status and has been recommended for universal use by the General Conference on Weights and Measures.
This includes various units of measurement for various forms of radiation, such as curie, rad, rem, roentgen, gray, sievert, coulomb. Moreover, these come in different dosages such as tera (T), giga (G), mega (M), kilo (k), centi (c), milli (m), micro (µ) and nano (n).
mCi en Mbq
I limit myself to the units that I have dealt with during my medical treatment. These are Millicuries (mCi) and Megabecquerels (Mbq). Millicuries (mCi) are considered a general unit in the SI Unit system and are the English unit of measure for radioactivity. The Becquerels (Bq) are the unit of measurement used in Europe.
The International System of Units for Radiation Measurements states:
Common Units: Radio-activity: curie (Ci), Absorbed Dose: rad, Dose Equivalent: rem, Exposure: roentgen (R).
SI Units: Becquerel (Bq), Absorbed Dose: gray (Gy), Dose Equivalent: sievert, Exposure: coulomb/kilogram (C/kg).
For the sake of completeness, I also place the technical definition here as I found it on the internet, a lot of information on the website:
The International League Of Atomic Women:
A millicurie (mCi) Is a decimal fraction of the deprecated non-SI unit of radioactivity defined as 1 Ci (is 1 curie) = 3.7 × 10¹⁰ decay per second. One curie is roughly the activity of 1 gram of the radium isotope ²²⁶Ra.
A megabecquerel (Mbq) A becquerel (Bq) is the number of atomic nuclei that decay radioactively per second. 1 Bq = 0.0000010 MBq. Bq is the SI derived unit of radiation activity. The Bq is defined as the activity of a quantity of radioactive material in which one nucleus decays per second. The becquerel is therefore equivalent to an inverse second, s⁻¹.
My dosage of I-131
For the definitive treatment of my overactive thyroid gland, I was administered 18 Millicuries of I-131 in America at the time. This has been converted for the European unit of measurement into Mega-becquerels: 666 MBq. I didn’t know that at the time I took it. I didn’t know anything about radiation. The internist in America had told me that the dosage had been suggested by my endocrinologist in the Netherlands. Previously I had already had a thyroid uptake scan twice in the Netherlands, which is performed with I-123, which has a half-life of 13 hours.
In total, I received 18 mCi of I-131 and 1.357 mCi of I-123
Named After
The Millicuries are named after the double Nobel Prize winner Marie Curie (1867-1934), who has also named the word ‘radioactivity’. She worked with her husband and fellow Nobel Prize-winning inventor Pierre Curie. To describe the behavior of Uranium and Thorium, she based radioactivity on the Latin word for sunbeam ‘radium’ or ‘radii’.
The Becquerel is named after Antoine Henri Becquerel.
Radium-emissions Standard
Marie Curie also won the right to define an international standard for radium emissions. Such a standard was essential for an efficient radium industry and uniform medical applications. The measure she introduced was accepted by the international scientific community, which called it the ‘Curia’.
However, she and her husband had to pay for their ‘magical’ invention with death later in life, because they and many others, unaware of the side effects of radioactive radiation, had handled it carelessly. In a letter she wrote to her cousin just before her death in 1886:
“My plans for the future? I have none….I mean to get through as well as I can, and when I can do no more, say farewell to this base world. The loss will be small, and regret for me will be short….”
For me, as the author of this article and an expert by experience, it has a kind of nostalgic value to read this, and the sentiment is not entirely alien to me. I also try to make the most of every day and enjoy all the beautiful moments that life offers. Even though the personal injury caused by radiation is difficult for me to bear on a regular basis (every day), there is still a lot worth living for.
In part 3 I will further discuss the consequences of radioactive radiation through nuclear energy for nature and our society.
RAI 1 – MY STORY
RAI 2 – WHAT IS IT?
NEXT:
RAI 3 – NUCLEAR ENERGY
Back to MENU
En dan ter afsluiting van deel twee nog even het motto van de website van The International League Of Atomic Women:
“We may as well move forward, because we can’t go back”.
© 2024 | Margreet Wilschut – Atomic Woman
Bron: www.margreetotto.net